
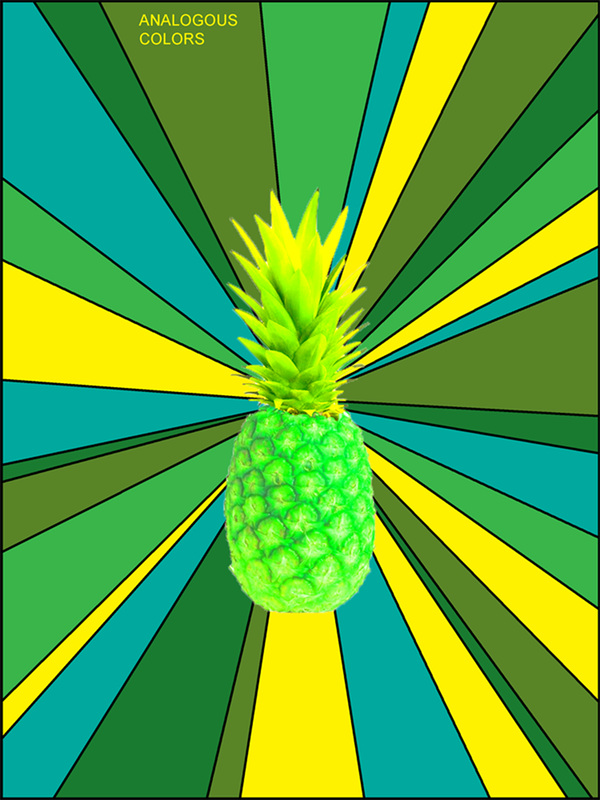
2.2 The Use of Neon Colors in the 1980s and OnwardsĮverything You Wanted to Know About Neon Colorsīright neon colors are electrifying and vibrant, adding energy and intensity to designs.2 Neon Colors Used in Art Through History.1.3 What Do Bright Neon Colors Signify?.1.2 The Difference Between Fluorescent and Neon Colors.1.1 The History of Neon Colors and Lighting.1 Everything You Wanted to Know About Neon Colors.Because of its stable octet, the electronegativity and electron affinity of neon approaches zero. Solid neon forms a crystal with a closely packed cubic structure. This is why neon lighting is used in cold regions and for aircraft and airports. One of the more interesting facts about neon is that the light emitted from ionized neon can pass through water fog.These are not neon signs even though many people commonly assume they are. Other colors of lights are produced by coating the interior of the glass with phosphors. When low-pressure neon gas is electrified, it glows reddish-orange.
NEON COLORS DEFINITION SKIN
The liquid can cause immediate frostbite to exposed skin or mucous membranes. Because of its high refrigeration capacity, liquid neon is used in cryonics to freeze corpses for preservation or for potential revival in the future. Neon is 40 times more effective as a refrigerant than liquid helium and three times better than liquid hydrogen. The liquid form of the element is a cryogenic refrigerant. It is also used in helium-neon lasers, masers, vacuum tubes, lightning arresters, and high-voltage indicators.

If a balloon is filled with neon, it will rise.

In fact, there are no known stable neon compounds, even though some other noble gases have been found to form chemical bonds. It belongs to the noble gas element group and shares the property with other elements of that group of being nearly inert (not very reactive).
NEON COLORS DEFINITION FULL
The element is in group 18 of the periodic table, making it the first noble gas with a full octet (helium is lighter and stable with only two electrons). The first two valence electrons are in the s shell, while the other eight electrons are in the p shell.
Because it has a stable octet for its outer electron shell, neon atoms have 10 electrons and no net electrical charge. There are three stable isotopes of the element, with atoms having 10 neutrons (neon-20), 11 neutrons (neon-21), and 12 neutrons (neon-22).


 0 kommentar(er)
0 kommentar(er)
